Project: The Issue with Tissue
A call to action to increase the usage of donated tissue samples for research.
The Issue
Patient surveys, including the recent HRA/HTA public dialogue, indicate support for legitimate medical research using human clinical samples and data where donors are informed and given the choice to participate or not.
A use MY data report which followed our May 2018 workshop, “Your data, your control”, explored how patients, who want to make sure that their consented samples and data are used for medical research, can ensure that this happens.
The current estimate from the biobanking community is that only 15% of samples are used, leaving a huge 85% never used.
From a patient perspective uses such as this, where consent is obtained, is very much an expectation. Having only 15% of samples being used feels unacceptably low. use MY data members were also concerned when they heard about the practical difficulties of accessing samples and data and wanted to take a role in maximising appropriate research.
Once this was highlighted, patients were determined to seek change.

A patient-focused workshop
As a result of this, use MY data, the Medicines Discovery Catapult and Incisive Health facilitated a workshop in September 2019 to seek solutions and identify key areas for further campaign activity.
The workshop brought together stakeholders from patient groups, researchers, industry and tissue banks. The report captures the discussion from the day and our recommendations for improving the use of human tissue samples.
Developing a citation to highlight that research has used tissue donated by patients
At the workshop it was proposed, to highlight the beneficial uses of donated tissue, that patients should be acknowledged in research that has relied upon access to tissue samples.
We have already seen extensive adoption of the use MY data patient data citation. The citation was conceived and developed by use MY data and says "This work uses data provided by patients and collected by the NHS as part of their care and support". It has been widely adopted by data and research organisations, and recommended for adoption by the major research funders in the UK.
We thought this could be adapted to reflect the use of tissue samples.
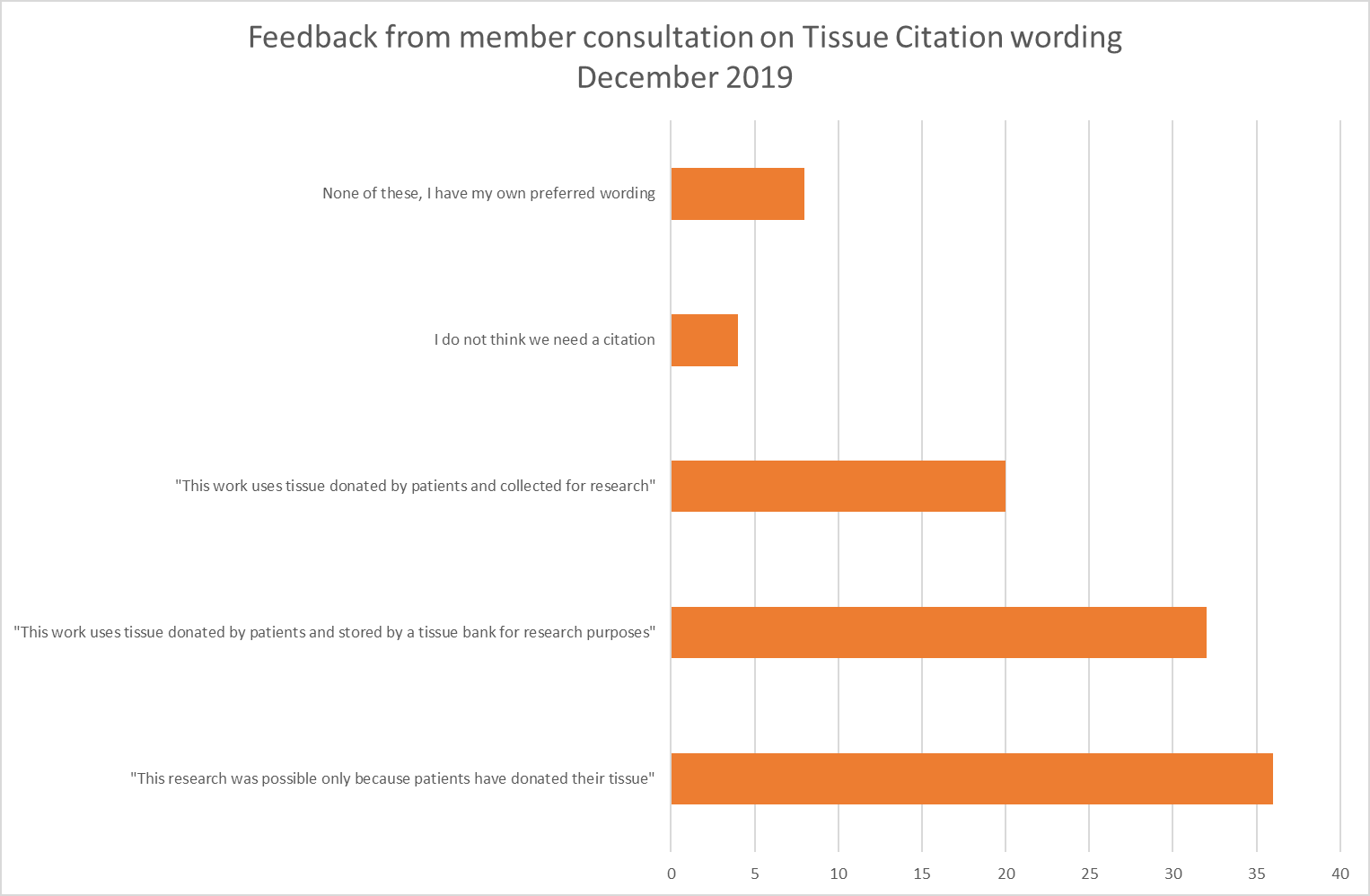
use MY data consulted its membership during November and December 2019, to choose a citation from a list of options that were suggested by members. After a period of voting, the most popular choice was:
"This research was possible only because patients have donated their tissue"
We will work with the co-authors of the report to plan for the wider uptake of the citation.
Final report and recommendations
As a result of the workshop, we produced a joint report which identifies 10 key areas of actions which needed to be taken by a range of stakeholders.
Our report "The Issue with Tissue: Recommendations for the use of human tissue samples" was published in January 2020, and is available here.

The recommendations in the report are designed for impact and aligned with the major stakeholders in this area. While improvements must be patient led, funding organisations have a unique opportunity to understand the perceptions and support the intentions of patients in this area, and to align their communications and delivery plans to the recommendations in this report.
This report is very much a call to action and is the start, rather than the end of the process. Over the next months we will work with the co-authors of the report to plan for the wider uptake of the citation and progress on all the points raised in the report.
This activity has been supported by a grant from Roche Products Limited. Roche Products Limited has had no control over the educational content of this activity
Tissue Access for Patient Benefit - COVID-19
The collection and usage of tissue is in particular focus, with the response to COVID-19. There is a growing recognition that, while samples are collected, they also need to be accessible to the research community.
Tissue Access for Patient Benefit (TAPb) is a University College London (UCL) and Royal Free NHS Foundation Trust initiative, which works closely with the UKCRC Tissue Directory and Coordination Centre.
The article 'TAPb, accelerates UK COVID-19 clinical trials by providing patient samples' highlights:
“Since the coronavirus outbreak TAPb has focused on collecting and delivering samples from COVID-19 patients, supporting scientists in research related to COVID-19 and the design of vaccines…
With many UK Biobanks having limited capabilities during this time, some researchers are struggling to obtain the samples needed to advance their work…TAPb has responded quickly and flexibly to the crisis and is the first UK tissue collection organisation to supply COVID-19 samples to researchers beyond their own institution.”
The article was published on 15 April and is available here.
UKCRC Tissue Directory and Coordination Centre – Report
The UK Clinical Research Collaborative Tissue Directory and Coordination Centre (UKCRC TDCC) has issued a briefing in support of a new Research Governance Survey Report by the Medical Research Council (MRC) Regulatory Support Centre which has highlighted that “Researchers need more support when it comes to sharing tissue samples and data”.
“When it came to running research, 25% of respondents said that obtaining data and tissue caused them moderate to severe difficulty…Difficulties in determining what legal frameworks applied to data/tissue sharing was also referenced…
Interestingly, when it came to issues with finishing research “sharing data /tissue” was the top cited research issue, referenced by 26% of respondents. About half the respondents from this group said that “sharing data or tissue was an issue as they were not sure whether the consent allowed sharing.”
The UKCRC’s briefing was published on 27 January and is available here.
The Issue with Tissue - next steps
Following a delay caused by the impact of COVID-19, the Secretariat is now working to resume use MY data’s The Issue with Tissue campaign - a call to action to increase the use of donated tissue samples for research. We are seeking external funding to assist with resuming the campaign and will provide an update when we know whether this is successful.


